IFA database
How are product data transferred from the supplier to the data recipients?
IFA GmbH, as neutral and central service organisation for standardised and quality assured information, offers its services to the different participants within the healthcare market.
The following diagram shows the flow of data from first publication into the IFA database to the usage of data recipients’ IT systems.
For more detailed information on the flow of data and IFA GmbH’s tasks, please refer to the following document IFA Database and IFA Information Services.
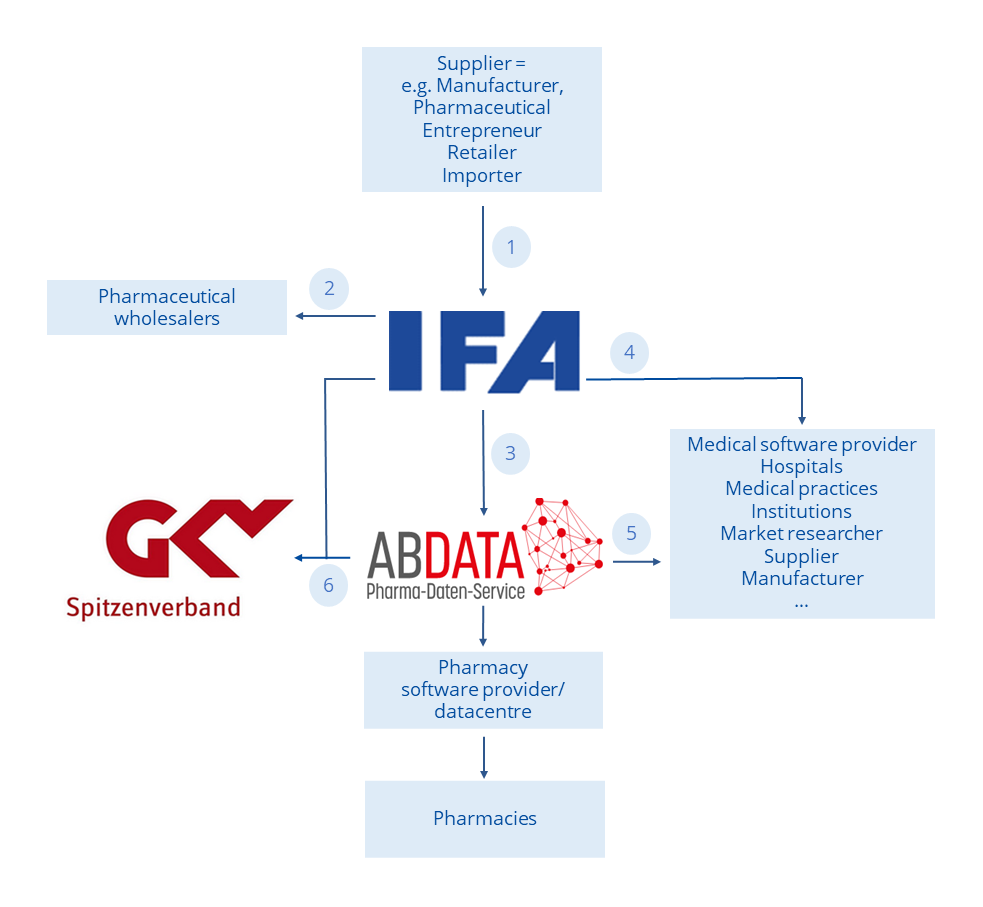

- Receiving, checking and recording supplier notifications in the IFA database
- Output of IFA information services to the pharmaceutical wholesalers
- Output to ABDATA Pharma-Daten-Service as part of the cooperation with IFA
- Output to other data recipients (including medical software providers)
- Supplementation of IFA information services by ABDATA Pharma-Daten-Service and distribution to pharmacy software providers and data centres as well as other authorised data recipients, in particular for data use in pharmacies and physician practices
- Joint issue of a product directory to the GKV-Spitzenverband (National Association of Statutory Health Insurance Funds) by IFA and ABDATA Pharma-Daten-Service; on behalf of the relevant manufacturer associations in relation with the notification obligations according to the framework agreement § 131 SGB V
IFA Guide
Table of content
×



 IFA Guide
IFA Guide