Counterfeit protection
According to art. 54a paragraph 1 of the so-called European Falsified Medicines Directive 2011/62/EU (FMD) and the altered guideline 2001/83/EG, prescription-only medicinal products have to bear security features in general. These make it possible to identify packs and help prevent fraud.
Details regarding characteristics and technical specifications of the individual distinctive feature for the security features can be found in the delegated act (EU) 2016/161 of the European commission from 2 October 2015. These were published on 9 February 2016 in the Official Journal. All participants of the pharmaceutical legal distribution chain must implement these prerequisites since 9 February 2019. Since the deadline, medicinal product obliged to the FMD must not be marketed without bearing the security features.
The directive allows for an end-to-end verification system of all medicinal products bearing the security features to guarantee their identification and validity.
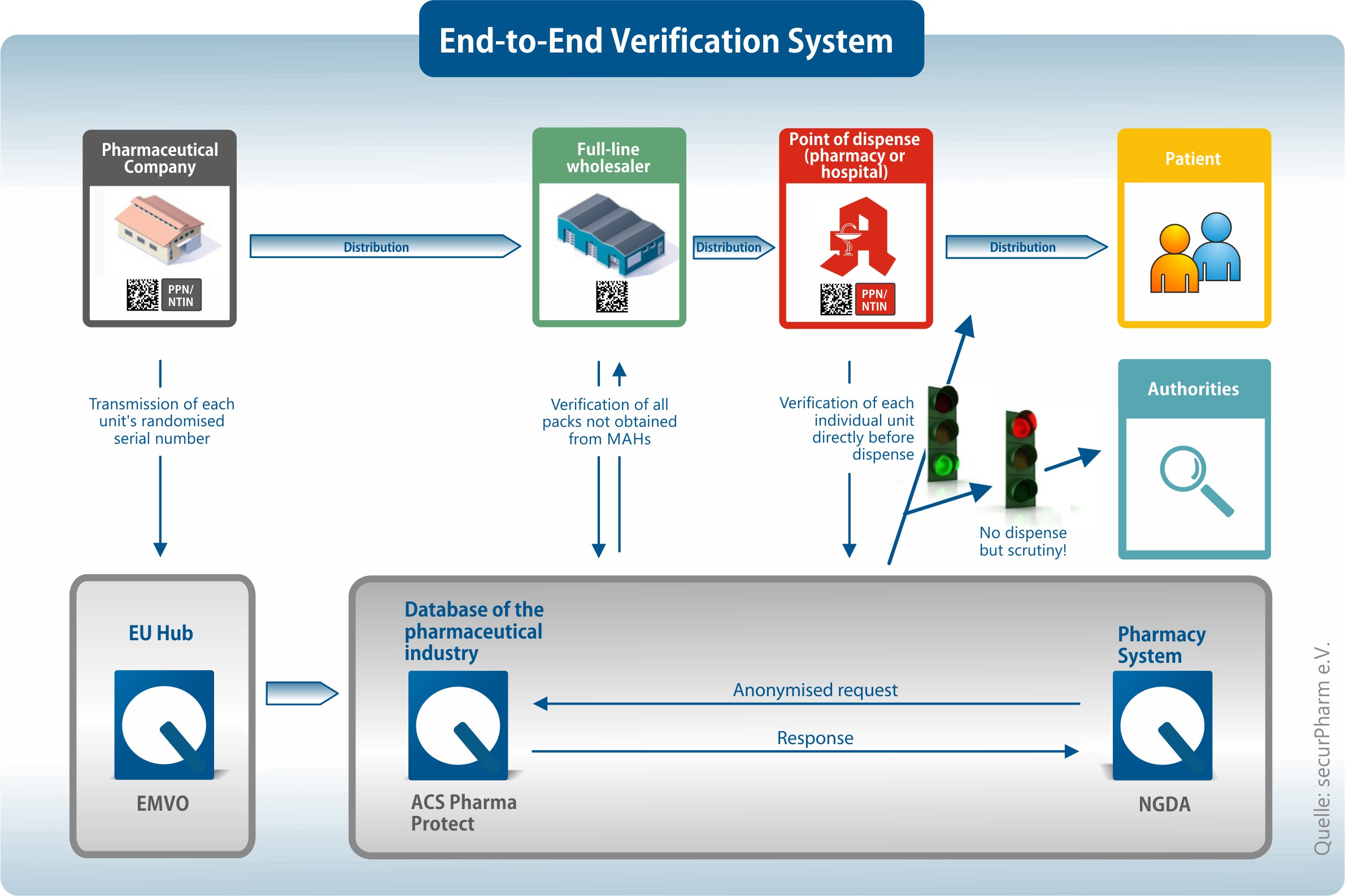

The necessary markings and coding of sale packs must be done according to securPharm’s coding regulations.
IFA’s specification PPN-Code Specification for Retail Packaging implements these regulations. It contains all identical elements of securPharm’s coding regulations.



 IFA Guide
IFA Guide