Global use of the PPN

Aided by the Pharmacy Product Number (PPN), every single national product number becomes world-wide unique.
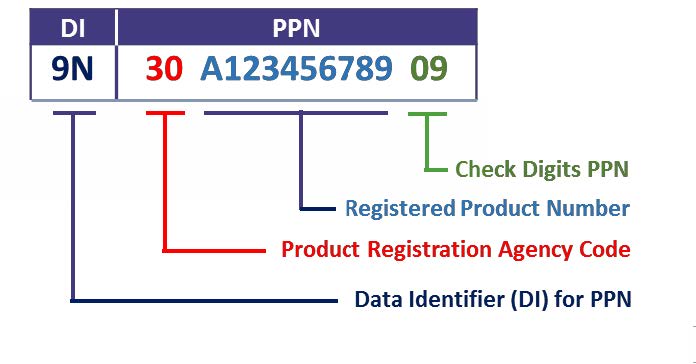
IFA as issuing agency assigns the Product Registration Agency Code (PRA-Code) for each national numbering system that exists in the pharmaceutical sector. With the PRA code as prefix, each national product number is transferred into an internationally unique product number (PPN). A 2-digit check-sum secures the PPN against faulty data input or exchange. Retaining the national product numbers and harmonising the different numbering systems eases the marking of sales packs and billing in the healthcare sector.
The especially standardised data designator “9N”, issued by ANSI MH10 Maintenance Committee, identifies the PPN in any data medium such as the Data Matrix Code.
Current information on the IFA Coding system and the application form for assigning a PRA code can be found here:
- Specification UDI Use of the IFA Coding System for MD
- PPN-Code Specification for Retail Packaging
- Application form PRA-Code
Request Form PRA-Code - Term&Conditions of Service



 IFA Guide
IFA Guide